

Christian Fuczik, Institut für Hanfanalytik
A guide to the current cannabinoids
Although we now know that over 200 cannabinoid compounds may be found in the cannabis plant genus, until a few years ago, tetrahydrocannabinol (THC) and cannabidiol (CBD) were the main compounds of interest to the cannabis community. Other cannabinoids have come into the spotlight due to creative product development by the cannabis industry. At the end of 2021, SSC (semi-synthetic cannabinoids) entered the European market and, with them, a multitude of other substances and abbreviations, making it challenging to keep track. This guide is aimed at interested parties who are professionally involved with cannabis and cannabis products or their users but have not devoted themselves to the study of chemistry or pharmacy. It intends to provide a brief overview of the currently relevant cannabinoids.
This list does not claim to be complete. It mentions those cannabinoids that have proven relevant in our daily work in the analytical laboratory and in discussions with our clients and partners.
In our lab, we have seen that classifying cannabinoids according to specific criteria greatly facilitates understanding. We categorize cannabinoids according to their origin:
Another section is devoted to the characteristics of the various cannabinoids in product analysis or drug testing.
Cannabinoids 2023 Poster A0 (6,4 MB)biosynthesized in the plant
The cannabis plant (Cannabis Sativa L.) bio-synthesizes the natural cannabinoids as carboxylic acids. The neutral forms with the desired biological properties are produced by decarboxylation with the addition of energy (light, heat, etc.). In the following, we always use the neutral form, including the carboxylic acid, if applicable.
The pentylcannabinoids are the most prevalent cannabinoids. Biosynthesis in the plant occurs through geranyl pyrophosphate and olivetolic acid. The alkyl side chains of olivetoids contain five carbon atoms.
CBD is produced in the plant by enzymatic conversion from cannabigerol (CBG). Numerous studies describe CBD as a substance for treating anxiety disorders, pain, cognitive and movement disorders, etc., on the subject. Anecdotally, successes are reported time and again. However, research is still underway to investigate the mechanism of action of the biological effects of CBD.
d9-THC is formed in the plant by enzymatic conversion from CBG. Like many pharmacologically active phytochemicals, it is presumably involved in the evolutionary adaptation of the plant against insect damage, ultraviolet light, and environmental stress. When consumed, THC is absorbed into the bloodstream and enters the brain, where it attaches to endocannabinoid receptors (CB1, CB2) in the cerebral cortex, cerebellum, and basal ganglia. In other words, those parts of the brain responsible for thinking, memory, pleasure, coordination, and movement. THC is covered by the United Nations Single Convention on Narcotic Drugs of 1961. It is, therefore, subject to the Narcotic Drugs Act, New Psychotropic Substances Act, or the respective national equivalent in most EU states in the summer of 2023.
CBG is only found in tiny amounts in cannabis plants, as it is immediately converted enzymatically into other cannabinoids (THC, CBD, CBC) during plant growth after biosynthesis. Targeted breeding has resulted in some varieties that hardly produce the enzymes needed for conversion, so CBG is still present in these plants when harvested.
CBC is formed in the plant by enzymatic conversion from CBG and possibly influences the psychoactive effect of THC and has an anti-inflammatory effect, which may contribute to pain relief. In mouse models, positive effects on breast cancer as well as anticonvulsant effects, have been shown.
Propylcannabinoids are so-called homologs of the pentylcannabinoids mentioned above. Their alkyl side chains have only three instead of five carbon atoms, but other than that, their structure is identical. Substances with the same structure but different alkyl side chain lengths are called homologs in chemistry. It is believed that, among other properties, the length of the side chain significantly influences the potency of the cannabinoid in question. Shorter alkyl side chain – lower potency; longer alkyl side chain – greater potency. The maximum potency of the respective cannabinoid is assumed to be at an alkyl side chain length of 8 carbon atoms.
Plants with elevated levels of propylcannabinoids are increasingly found in cannabis populations from China, India, Nepal, Thailand, Afghanistan, Pakistan, southern and western Africa.
CBDV is a non-intoxicating, mildly psychoactive homolog of CBD that is believed to have anticonvulsant properties. GW Pharmaceuticals is developing CBDV under the product code GWP42006.
CBGV is a homolog of CBG in which no psychoactive but analgesic and anti-inflammatory properties have been observed.
d9-THCV is a homolog of d9-THC. In studies, it has been shown to have neuroprotective effects, suppress appetite, control blood sugar levels, and have fewer side effects than THC. Its suitability for treating obesity and diabetes is being investigated. In Austria, THCV is categorized as a "new psychotropic substance" according to official information in June 2020 and is covered by NPSV Schedule II.
The cannabinoids categorized here are produced in the plant by natural aging processes caused by oxygen in the air, moisture, light, ultraviolet radiation (UV light), and heat during storage. Of course, these aging processes can also be intentionally induced or accelerated to produce these substances in larger quantities.
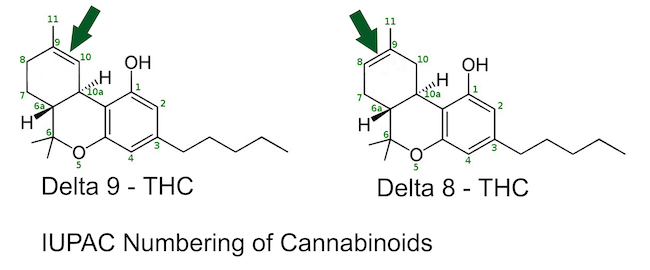
d8-THC is formed by rearranging the double bond from position 9 to the thermodynamically more stable position 8 d9-THC vs. d8-THC shown in IUPAC numbering It has similar psychoactive properties as d9-THC but to a lesser extent. During the production of THC from CBD, a greater or lesser amount of d8-THC is produced in addition to d9-THC, depending on the reagents used in catalysis.
CBN is formed by the oxidation of THC. The longer THC-containing products are stored under oxidative conditions, the higher the concentration of CBN. Even though CBN shows a slightly psychoactive effect, it is not listed as a "New Psychotropic Substance" in Austria and Germany. This fact and the fact that HHC products were banned in many European countries in the spring of 2023 led to an increased supply of commercial CBN products.
CBTC is a non-psychoactive cannabinoid that has also been found in plants of other genera. Since it is rarely found naturally and only in low concentrations, it is suspected that CBTC may be an aging or degradation product. In a 1984 study, CBTC was able to reduce eye pressure in animal experiments.
CBT is an oxidation product of tetrahydrocannabinol, which has been detected both in trace amounts in cannabis plants and as a metabolite in cannabis users. Reliable and citable sources on the pharmacology of CBT are not known.
CBL is a degradation product of cannabichromene. As of summer 2023, there is hardly any reliable scientific knowledge about CBL.
CBE is a degradation product (pyrolysate) of CBD and is therefore found in the smoke of cannabis cigarettes. A 1988
Japanese study found it to be a metabolic product of CBD in guinea pigs. Due to the low scientific interest in CBD
pyrolysates and guinea pig metabolites of CBD, there is little robust scientific knowledge on this as of summer 2023.
CBE is mentioned in this list because we repeatedly receive samples in the laboratory that the sender assumes would
contain significant amounts of CBE. CBE shows similar chemical behavior to d9-THC in classical analysis of hemp
products. Therefore, advanced laboratory methods such as Cannabis Advanced Analysis are necessary for correct
identification. See chapter:
Cannabinoids in the chemical laboratory
– section:
Analyzing conversion products
SSC refers to a group of substances converted from natural cannabinoids using simple chemical processes (mostly hydrogenation). Therefore, products containing SSC are called conversion products. SSCs usually have the typical properties of cannabinoids (e.g., psychoactivity) but were not regulated by law when they appeared on the European market (2021/2022). In the meantime, many psychoactive SSCs are subject to legal regulations in European countries (Austria, France, Poland, Finland, etc.) and may no longer be traded. Scientific studies on the mechanisms of action of SSCs are still lacking.
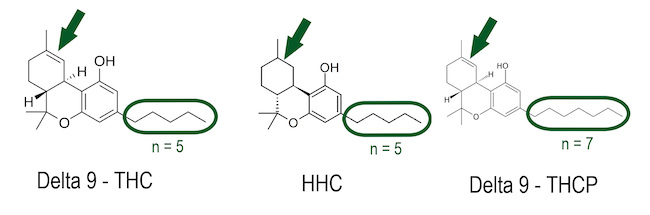
HHC is a psychoactive derivative of THC. It is produced by hydrogenation of THC, retaining the basic structure of THC Chemical structures d9-THC vs. HHC vs. THCP Although often presented this way, it is not biosynthesized in the cannabis plant. Those two publications that found HHC in minimal traces in heavily aged plant material also tend to suggest an aging process. Within the EU, the EMCDDA 2023 "Technical Report: HHC and related substances" is of legal relevance, which excludes biosynthesis and, thus, the natural origin of HHC.
HHCO is an HHC derivative. It is believed to act as a pro-drug, similar to THC-O, which is hydrolyzed to HHC in the body after consumption. Scientific studies on HHCO are still lacking.
HHCP is a very strongly psychoactive substance. It is assumed that the more substantial psychotropic effect compared to HHC is mainly due to the extended alkyl side chain (7 carbon atoms instead of 5 as in HHC).
CBND is a psychoactive cannabinoid. In American forum posts, it is heralded as one of the successors of HHC, which is why we have categorized it as SSC. Chemically, it was first found in low concentrations in hemp flowers in 1974. It is mentioned in only a few scientific publications. Its structure corresponds to a complete aromatization of CBD, analogous to the structures of CBN to THC. This would rather speak for a degradation product. Apart from its psychoactive property, there are no data on the mode of action of CBND.
CBN-O is a derivative of CBN. It is believed to act as a pro-drug, similar to THC-O, which is hydrolyzed to CBN in the body and then exerts its effects as CBN. Scientific studies still need to be included.
H4CBD is obtained by catalytic hydrogenation of CBD. In the wake of the international price drop of CBD, starting in 2022, a CBD product with a higher price was created and offered as H4CBD under a new name. H4CBD is believed to have the same properties as CBD but to a greater degree. Scientific studies are still lacking.
THC-O is the acetate ester of THC. It is a metabolic pro-drug, i.e., hydrolyzed in the body to THC, which only develops its effect as THC after hydrolysis. The strength of the psychoactive effect depends on whether it was synthesized from d9-THC or d8-THC.
THCP is a potent psychoactive synthetic homolog of THC. It is believed that, like HHC, the extended alkyl side chain (7 carbon atoms instead of 5 as in THC) results in a stronger psychotropic effect than THC. Isomers with the double bond in position delta 8 and delta 9 are probably.
SSC products may be contaminated with either extraction residues or synthetic by-products and may contain traces of heavy metals originating from the catalyst used for hydrogenation. Here are some substances involved in the production processes of semi-synthetic cannabinoids. Depending on the process and substance, they are desirable intermediates or undesirable byproducts and are sometimes still present as impurities in finished products for end users. These byproducts get the most attention as an unexpected laboratory result, both in product testing as part of quality assurance and in post-consumer drug testing.
Depending on the manufacturing process, d6a10a-THC may be an intermediate or by-product in the production of HHC. Depending on the ratio of the two enantiomers (R, S), d6a,10a-THC has little to hardly any psychoactive effect.
d8-iso-THC and d4(8)-iso-THC are formed during the cyclization of CBD to THC.
d10-THC is a THC isomer with approximately 30-40% of the psychoactive potency of d9-THC. D10-THC has rarely been detected, and then only as a trace component, in cannabis plants. Therefore, d10-THC is presumably a degradation product similar to CBN. However, it is also frequently found as an impurity in synthetic d8-THC produced from CBD. For this reason, we have categorized d10-THC as a byproduct. From another perspective, it can also be considered a degradation product or SSC.
d11-THC is a synthetic isomer of THC that is produced, among other substances, as an impurity in the production of dronabinol (d9-THC). It can be synthesized as SSC from d8-THC in several ways. In experiments with mice, d11-THC has shown a psychoactive strength of only about 25% compared to d9-THC. Chemically, d11-THC is no longer a "tetrahydro"-cannabinoid because the double bond is outside the name-giving ring between positions 9 and 11. See Figure 1 IUPAC numbering. When viewed from the ring, the double bond is on the outside (exo), and only 5 carbon atoms are hydrogenated in the ring itself. Therefore, the compound could be called pentahydrocannbinol.
SC or cannabinoid mimetics were sold in the mid-2000s as spice or herbal blends. When they appeared on the market,
hardly any legal regulations were in place. Among other substances, SC was sold under the name "legal highs".
These compounds mimic the body's biologically active endocannabinoids, which can amplify or inhibit nerve signals. The
strength of the effect varies considerably from substance to substance, and the risk for severe intoxication is more
likely compared to THC.
When they first appeared on the market, there was little legal regulation, hence the term "legal highs." A flourishing
trade quickly developed, starting with Chinese producers. Individual chemical functional groups were innovatively
varied to bring new substances with varying desired (and undesired) effects onto the market. SCs have subsequently
been categorized as either narcotics/addictive drugs or "New Psychotropic Substances – NPS," depending on the
substance and country. Accordingly, laws were enacted, allowing entire groups of substances to be regulated rather
than just individual substances. In their structure, SCs differ significantly from natural cannabinoids and also SSC
and can be easily separated from each other when analyzed. Several hundred SC have been seen on the market in Europe.
Laboratories of security agencies have created their own task forces to identify SC.
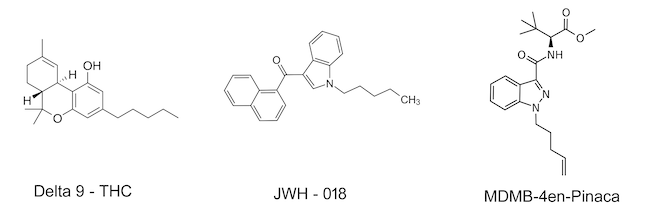
Chemical Structures of d9-THC vs. JWH-018 vs. MDMB-4en-Pinaca
John W. Huffman synthesized a series of chemical compounds, named after his initials, that affect the endocannabinoid system. JWH-018 was one of the first SC representatives to enter the market as a "legal high." The affinity for the cannabinoid receptor (CB1) of JWH-018 has been reported in studies to be five times greater than that of THC.
AB-PINACA is a compound first identified in Japan in 2012 as a component of synthetic cannabis products. Initially developed by a pharmaceutical company in 2009 as a pain-relieving drug, it acts as a potent agonist for the CB1 receptor.
MDMB-4en PINACA is a synthetic cannabinoid that has been circulating in Europe since 2017, and its prevalence has been increasing significantly since mid-2019. Due to its high potency, it can pose a significant risk for severe poisoning.
Analysis of Cannabis Products and Drug Testing after Consumption
Producers and distributors often state that their latest SSC product is "THC free" and that consumption would not be detectable in a drug test. Therefore, one would not have to fear any consequences in case of consumption. However, this is wrong in both senses. In the past, most SSC products have turned out not to be "THC-free," and drug tests have been unexpectedly positive for THC. To explain, it is essential to understand that drug testing is done in different ways and for other purposes. Therefore, for a result expectation of a cannabinoid in a drug test, one should know the drug test variants.
Forensic toxicological analysis for the assessment of driving ability in case of suspected impairment
Every European country has a legally regulated procedure to analyze and evaluate the concentration of THC and its metabolites. In the case of the other psychoactive cannabinoids, it can be assumed that the laboratory will detect and include them in the experts’ assessment as a "new psychotropic substance."
The first step in drug testing from urine is screening with immunological tests and automated analyzers. There are various manufacturers of these immunological reagents. The reactivity of the corresponding product with the respective cannabinoids varies slightly. THC and its metabolites are always detected. For the other cannabinoids, this depends on the manufacturer.
Basically, one should expect all substances with a THC-like structure (listed here with the pattern: dX-THC and HHC) to show a positive reaction in a THC test.
Depending on the question and the medical history, positive results are confirmed with another method in a second step. If no evidence of d9-THC or its metabolites is found in the analysis with the second method, but, e.g., d10-THC or HHC is found, it is for the respective institution to decide whether a requirement was violated or if the proof of abstinence was provided.
Various studies and countless self-experiments have shown that CBD and CBG are not detected with immunological THC tests. However, one should remember that several CBD products, especially those advertised as "full spectrum" or "broad spectrum," may also contain THC. The THC content in these products is too low to cause intoxication but may result in a positive THC test.
Synthetic cannabinoids (SC) are detected with specific tests. They do not appear in THC tests.
Since there are various manufacturers of strip tests of very different quality on the market, no statement can be made about the expected results of SSC in strip tests.
With the advent of conversion products, the number of possible cannabinoids in hemp products has more than doubled. The usual determination of 8 to 12 phytocannabinoids or their degradation substances is no longer sufficient to characterize product samples adequately. To adequately document the presence or absence of marketed SSCs in a sample, it is necessary to use additional methods. In addition to the HPLC-DAD method, which has always been used, conversion products must be analyzed by mass spectrometric techniques (GC/MS or LC/MS).
We have developed the Cannabis Advanced Analysis in our laboratory, which combines the advantages of those methods to identify all contained cannabinoids correctly and to make reliable statements about the legality and quality of a conversion product.

Christian Fuczik is a graduate of the HBLVA Rosensteingasse Vienna, where he graduated in 1987 with a major in technical chemistry. Since 1989, he has been involved in the chemical analysis of psychotropic substances in various positions. He is the Founder and Owner of Drogentest Wien and the Instituts für Hanfanalytik , where around 1,000 companies from all over Europe have their products tested for quality and legality.